
Laboratories
Institute
|
Chemistry is an experimental (and theoretical) science and art which is hardly ever performed at the beach (although the idea doesn't sound too bad); one needs laboratories for that. Our laboratories are part of the Institute of Inorganic Chemistry which is depicted on the figure below:

As you may have guessed already, the buildings of RWTH Aachen University are not located on a separated campus (well, some institutes are) but most of the university buildings are spread over the northwestern part of the city of Aachen. The Institute of Inorganic Chemistry (IAC, in short) is located on top of the "Königshügel" (king's hill), very close to the main RWTH building and also not far from the very center of Aachen city. The upper picture shows an outer view of the IAC building taken from its inner courtyard.
| Here is another picture of the IAC building which hosts both the chemistry library as well as the largest chemistry "Hörsaal" (lecture hall) called "AOC". If you intend to listen to a particular lecture, chances are pretty high that you find yourself inside AOC at some time. More pictures of this (and other) lecture halls are also provided in the teaching section.

Despite the fact that the building was constructed in the early 1950s, it underwent a complete reconstruction at the beginning of the 21st century and now provides optimum laboratory conditions for all kinds of state-of-the-art inorganic chemistry. Needless to say, the entire building is easily accessible for disabled people, too.
|
Synthesis
|
There is a tricky synthetic problem within solid-state chemistry: at room temperature, atoms (and ions) in solids are not very mobile such that it would take ages to wait for most solid-solid reactions to complete. That's a major difference if compared to molecular chemistry which usually depends on solvents; in these, many molecules are easily dissolved and may react rapidly to form new molecules. Unfortunately, there are only very few solvents for solid-state materials and, even worse, many solid-state phases do not exist as liquids or gases. Thus, how do we force solid-state materials to react? Heat 'em! Solid-state chemists heavily rely on high-temperature routes (and ovens, see figure on the right), the classical, "ceramic" way to synthesize new compounds. Rumor says that most solid-state chemists are fire-proof and can easily touch red-glowing iron but that may be an exaggeration...

Of course, solid-state chemical syntheses occuring around 1000 K cannot be performed in glass beakers; to nonetheless encapsulate the corresponding chemicals we seal them (that's just one way to do it) inside inert-metal containers (tantalum, for example) and close the containers by arc-welding (see upper figure, showing Dr. Boniface P. T. Fokwa and Dr. Wuping Liao).
| Here is a typical high-temperature oven of ours. It may be used for chemical reactions that occur between, say, 700 and 1000 K. In same cases, even these reactions have to be protected against the laboratory atmosphere (which is contaminated with 20% oxygen and traces of water), and this is easily done using an inert-gas atmosphere, for example elementary argon.
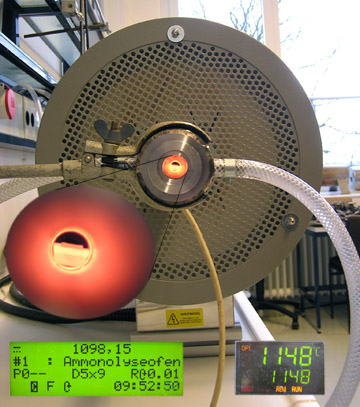
Nonetheless, there are many other (sometimes even smarter) ways to make solid-state materials react at moderate conditions. Come and visit our laboratories for obtaining more information on how to accomplish this!
|
X-Ray Diffractometry
|
Because most solid-state materials exhibit good crystallinity, diffraction methods are the golden way to fully characterize the materials under study; as a solid-state chemist, you can easily live without an NMR instrument (who needs such machines anyway?) but the permanent use of high-performance X-ray diffractometers is absolutely mandatory. Like in many other solid-state laboratories around the globe, our freshly synthesized solid-state materials are characterized (in terms of structure and composition) via X-ray diffractometry. Fortunately, we have access to state-of-the-art powder and single-crystal diffractometers at our own institute. When it comes to neutron diffraction, we hold strong ties to neutron facilities all over Europe.

This G670 Imageplate Guinier diffractometer (see above figure, made by Huber, Rimsting) equipped with a Johanson Ge monochromator for Cu-Kα1 radiation is used for most measurements.

| 
In order to study the behavior of solid-state materials at low or high temperatures, we use the following X-ray powder diffractometers. The upper picture shows the G645 Guinier diffractometer (Huber, Rimsting) which is equipped with a closed-cycle refrigerator working between 8 and 300 K. The latter is shown in greater detail in the figure below, right. For the G645, either a scintillation counter or a position-sensitive detector (PSD) may be utilized for the detection of Bragg peaks. The figure at the left bottom shows the high-temperature Guinier diffractometer G644 under operation while the sample is heated up to a temperature of 1200 K; this is pretty hot already.

In case we are lucky, new compounds may directly grow as single crystals, either by pure accident (these things happen!) or after tremendous experimental effort. Single crystals go straight to the (digital) CCD X-ray single-crystal diffractometer (see left figure) and yield their non-overlapping Bragg peaks. Eventually, these data are mathematically back-transformed into a three-dimensional structure model - with unmatched atomic resolution. In fact, the accuracy of such models based on well-resolved X-ray single-crystal data is superior to those of any other method.
|
Computational Chemistry
|

In case all supercomputing centers would blow off in a gigantic fireball, we could still perform many numerical calculations using those machines hidden in the institute's basement; these are standard computers, more or less, similar to your own PC. Have a look, however, at our brand-new home-made Linux cluster (see figure above) which was recently envisioned and assembled, skillfully so, by Dr. Bernhard Eck sitting in front. This particular computing beast made for the purpose of parallel processing contains 18 CPUs (or 36 cores) and runs at a speed of 42 Gigaflops! For less than Euro 15.000, that's pretty fast! Thank you, German Science Foundation - and thank you Bernhard, too.

The NEC SX-8 number cruncher at the Stuttgart HLRZ; we need those machines, yes we do.
| It sounds silly but even if there was no numerical quantum chemistry, supercomputer manufacturers would have to invent it because (quantum-chemical) computational chemistry eats CPU time like candies! No matter how fast the computers, we will always be able to challenge any supercomputer by slightly increasing the complexity of the chemical problem; there is never enough CPU time available (because chemistry is a many-body science)! That's the sad part of the story.
Nonetheless, we enjoy the privilege to have relatively easy access to CPU time wherever it may be available. Smaller calculations are typically performed on our own Linux cluster (left picture) whereas larger calculations ("jobs") have to be sent to various supercomputing centers and are crunched at these. First, at Aachen the RWTH computing center offers its Sun Fire SMP cluster and its Opteron cluster which we gladly use. Second, the John von Neumann Institute at Jülich lets us benefit from their IBM p690 cluster and the wonderful IBM Blue Gene/L supercomputer (see figure below). Third, we also exploit the Stuttgart High-Performance Computing Center and its magnificent NEX SX-8 machine (see figure left, bottom). Depending on the problem, calculations may run for seconds, minutes, days, weeks, or months.

The IBM Blue Gene/L machine at Jülich; powerful, yep, but still light years behind HAL9000 (shipping somewhat delayed), see below:

|
Library
|

The chemical library is shared with both the neighboring Institute of Organic Chemistry and the Institute of Physical Chemistry. Our library provides students and scientists with a huge survey of books and journals over a total of three floors. In addition, you may also order journals online. Because of its quiet and friendly atmosphere, you will not only find chemistry students here.
Website of our library
| 

|
Location
Aachen is a medium-sized German town in the heart of Europe located at the (former) borders with Belgium and the Netherlands. In fact, Aachen is Germany's most western town (good morning, America, how are you?), and among the ca. 300,000 Aachen inhabitants there are more than 40,000 students. Thus, Aachen appears to be a very "young" city to many people when they are taking a walk through its very center. Historically, however, Aachen is quite old since it was founded by the Romans about 2,000 years ago, simply due to the presence of hot springs. At that time, Aachen served as a military bath for the northern Roman empire, allowing the poor Roman soldiers to cure their suffering bodies, and Aachen was called "Aquis Grana" in the first century. Even today, you can take a cure at Aachen because the hot springs are still there!
In 768 Carolus Magnus, a German king which you might know under the French name "Charlemagne" but which we Germans call "Karl der Große" was coronated at Aachen, and Aachen became the center of his kingdom; in the sequel, Karl der Große also became Emperor of the Holy Roman Empire. In addition, Karl der Große built the Palace Chapel which then turned into the Aachen Cathedral. In case you are a Roman catholic, you (should) know Aachen anyway.
[Source: aachen.de for more detailed information about the history of Aachen]
According to the decision of Emperor Wilhelm I., the construction of the first building of our university was started in the year 1865 already. Five years later, the "Königliche Rheinisch-Westphälische Polytechnische Hochschule" was opened, the official birth of RWTH (Rheinisch-Westfälische Technische Hochschule) Aachen University as it is called nowadays. Note that the German word "Hochschule" is a synonym for "University", and it has nothing (nothing, really) to do with the Anglo-Saxon "High School".
[Source: RWTH Archiv for more detailed information about the history of RWTH Aachen University]
Among all German universities, RWTH has the highest national as well as international reputation when it comes to engineering science. Whenever you witness "German engineering" from abroad, chances are extremely high that this particular machine (car, aircraft, gadget etc.) has been constructed by someone who graduated from RWTH.
 Google Earth view of our Institute Google Earth view of our Institute
You need Google Earth (Freeware, external link) to view this file.
|

 Impressum und Datenschutzerklärung (Imprint and Privacy Policy) |
Administration |
Print
Impressum und Datenschutzerklärung (Imprint and Privacy Policy) |
Administration |
Print
 Google Earth view of our Institute
Google Earth view of our Institute